AI-driven pharmacometrics — PopPK, PBPK, and QSP — for precise clinical decisions

|
A new company has emerged in Korea, combining AI and modeling technologies for the first time.
While numerous AI-driven drug development companies exist, few truly understand core clinical development areas like PK/PD and apply them in real-world drug development. APLUS Simulation, officially launched late last year, targets this gap.
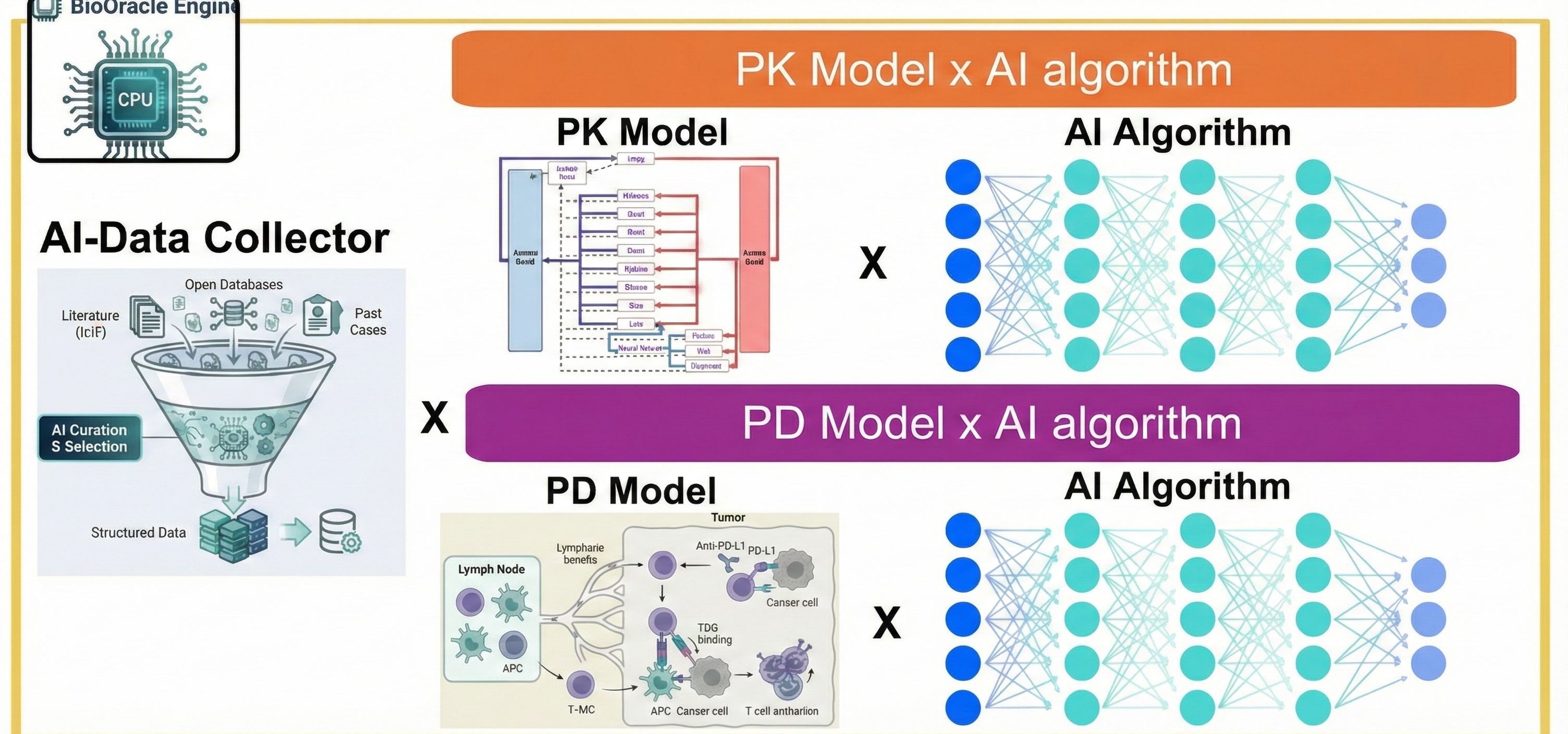
APLUS's core focus is integrating pharmacometrics-based modeling with AI technology. Building upon traditional pharmacometrics methodologies—PopPK, PBPK, and QSP centered on PK/PD—the company introduces new approaches by incorporating AI algorithms that add distinct value, ultimately enhancing clinical predictability and utility throughout drug development.
The company is developing computer-based AI-modeling software to support critical development decisions: AI algorithm-based automated data extraction and engine creation, animal-to-human translation prediction using AI and modeling, and ultimately, drug exposure and efficacy prediction across clinical trial phases.
Though newly launched, APLUS has deep industry roots. Co-CEOs Soo Hyeon Bae (Ph.D.) and So Jin Lee (Pharm.D., Ph.D.) are both pharmacists who served as key consultant in pharmacometrics at Korea's first pharmacometrics modeling group. Dr. Bae gained experience across academia, public research institutions, and industry, including an FDA fellowship program. Dr. Lee was a clinical pharmacist in U.S. and a starting member of Korea's first modeling company and currently serves as adjunct professor at Sungkyunkwan University's School of Pharmacy.
Though newly launched, APLUS has deep industry roots. Co-founder and Co-CEO Soo Hyeon Bae (Ph.D.) and So Jin Lee (Pharm.D., Ph.D.) are both pharmacists who served as key consultants in pharmacometrics at Korea's first pharmacometrics modeling group. Dr. Bae gained experience across academia, public research institutions, and pharma industry, including an FDA fellowship program. Dr. Lee was a clinical pharmacist in the U.S. and a founding member of Korea's first modeling company and currently serves as adjunct professor at Sungkyunkwan University's School of Pharmacy. Together, they bring decades of combined expertise in pharmacometrics modeling and clinical pharmacology to APLUS.
APLUS's greatest strength lies in its AI-integration approach—using AI not as a black-box replacement for modeling, but as a tool to enhance mechanistic models that connect biological mechanisms and clinical data through equations. AI is selectively applied where clinical utility is needed: data extraction, identification of key factors affecting drug exposure and efficacy, model refinement, and prediction accuracy improvement.
Current projects focus on complex modalities including antibody drugs, bispecific antibodies, ADCs, and gene therapies, where understanding mechanisms of action is particularly critical and complex.
The company is already collaborating with multiple pharmaceutical and biotech companies as well as AI research teams. APLUS aims to provide practical value in drug development, deeply engaging with tailored solutions for drug development programs to ensure AI-modeling integration directly supports development decisions—ultimately increasing the efficiency and probability of drug development success.
This approach aligns with the regional government's recent emphasis on AI-driven drug development policy initiatives as well as the FDA's regulatory direction centered on NAMs (New Approach Methodologies), positioning APLUS at the forefront of the evolving landscape of AI and NAMs-based drug development.
-
01 디티앤씨알오, 유진PE로부터 200억 규모 투... -
02 아미코젠, 인도 대리점 Aruni 통해 IEX 레진... -
03 아미코젠, 신규 인간 히알루로니다제 비임상... -
04 미간ㆍ눈가주름 개선용 액제 FDA 허가심사 개시 -
05 Meet APLUS: Korea’s First AI·Modeling Spe... -
06 ' 빠른배송'에 하나 더, 이커머스 대전 심화 -
07 한국유나이티드제약, ‘개량신약 60%’ 정조준... -
08 제약업계, 노동계 우군 확보… 약가 개편 대... -
09 복지부 품에 안기는 국립대병원, R&D·인프라... -
10 핀테플라, 드라벳 증후군 미충족 수요에 답...











